WP3
Work Package 3 (WP3): preclinical data on hyperthermic radiosensitisation
Aim of WP3 is to increase our understanding and to quantify the multiple synergistic effects of hyperthermia when combined with radiotherapy treatment. Clinically relevant questions include the effect of different sequence and different time intervals between radiotherapy and hyperthermia on the sensitizing effect of hyperthermia. This task is challenging due to the complex pleiotropic nature of hyperthermia, its many molecular and physiological effects include inducing heat shock response, reduced DNA repair, tumor growth, induced immune response, changes in vasculature and blood flow and induction of hypoxia. All these effects display different temperature-dependence and different dynamics and time scales for tumor and normal tissue responses.
Research in WP3 will use in vitro and in vivo tumor and normal tissue models to assess the effect of the main contributing effects for different radiotherapy and hyperthermia schedules for 39<T<43°C. Four ESRs will address these questions by developing clinically realistic models reflecting multiple mechanisms based on data obtained from systematic, carefully designed in vivo studies where multiple relevant end-points are quantified in normal tissue and multiple tumor sites.
ESR1 will focus on heat shock response and tumor tissue damage after radiotherapy and hyperthermia,
ESR2 on normal tissue damage,
ESR3 on the role of vascular, blood flow and hypoxia response,
ESR4 on the immune response
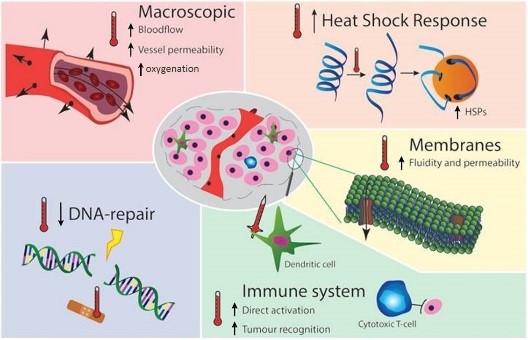
Figure 1 summarises the molecular and physiological mechanisms of hyperthermia-induced radiosensitisation. Hyperthermia at 39°C<T<42°C improves perfusion and reduces hypoxia, thus sensitising for Radiotherapy, at T>42°C vascular collapse occurs resulting in substantial direct hyperthermia-induced cell death, especially in already oxygen and nutrient-deprived hypoxic regions. Hyperthermia at T>41°C blocks homologous recombination DNA repair by degrading BRCA2, this temporary (~4 hr) DNA repair deficiency can be exploited to sensitise tumors to DNA damaging modalities e.g. RT. Hyperthermia at T>42°C restores p53, the apoptotic pathway which is disabled in HPV positive tumors like cervix cancer. Hyperthermia is an effective immunomodulator, contributing to activation of the innate and adaptive immune systems, effectively resulting in in situ tumor vaccination. Hyperthermia-induced HSP’s cause thermotolerance, reducing Hyperthermia effectiveness for ~3 days after Hyperthermia.
