Research
Background
preclinical, technical and clinical status of hyperthermia
Preclinical research identified multiple biological mechanisms responsible for the clinical HT effects (Figure 1).[1]
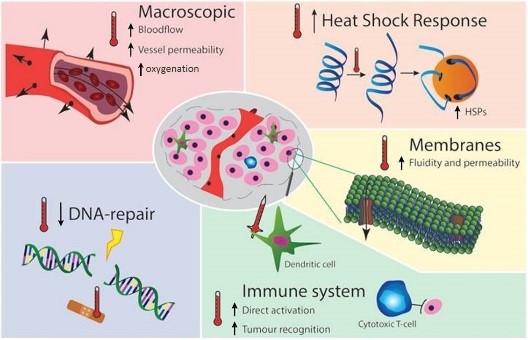
HT at clinically obtainable temperatures (40-44°C) causes protein, DNA and membrane damage, interferes with cell cycle, DNA/protein synthesis and DNA damage repair [2], triggers thermo-tolerance and apoptotic pathways by heat-shock proteins (HSP)1. Macroscopically, HT causes systemic activation of the immune system[3] and changes in blood flow and re-oxygenation [4], boosting RT which is less effective under hypoxia[5]. Our understanding of the molecular basis, time-dependence and temperature dose-effect relations of some mechanisms, particularly on HT temporarily blocking DNA repair by BRCA2 depletion2 and its effect on the best timing of RT and HT[6] has increased. Similar advances increased understanding of the enhancement of immunogeniceffects of RT by HT3 which can result in less metastases after RT+HT treatment compared to RT alone [7]. HT is also directly active against hypoxic tumor cells, an effect additive and complementing to the tumor effect of RT. All HT mechanisms thus enhance directly or indirectly effectiveness of RT, most effects are temporary and reversible at 40-44°C: effectiveness of each mechanism depends uniquely on temperature, sequence and time interval between RT and HT, in tumor versus normal, and hypoxic versus normally oxygenated tissues, locally and also systemically.
A crucial next step is unravelling of the clinical contribution and timing/dose-effect relationships of major mechanisms by which HT synergizes and enhances efficacy of RT.
Introduction
Interest in thermal treatments is rising as raising temperature has profound biological and clinical effects. Mild hyperthermia (HT), heating tumors to temperatures of 40-44°C, is a powerful, clinically proven cancer treatment used to enhance the efficacy of radiotherapy (RT) and/or chemotherapy (CH). Clinical effectiveness of HT has been proven in randomised studies and HT is reimbursed in many European countries for important indications, e.g. RT+HT for recurrent breast cancer. However, the timing, sequence and temperature dosage of HT-based therapies can be further improved to realise the full synergy and clinical potential of HT in multimodal settings. Preclinical research has shown that a multitude of biological and biophysical mechanisms are responsible for the clinical HT effects, all with a different temperature-effect relationship and with different optimal timing between RT and HT. Optimal HT delivery thus requires knowledge-based personalised planning. Unravelling key mechanisms and optimisation of clinical RT+HT treatments requires a quantum leap in understanding and in clinical application only achievable by an integrative and holistic approach involving all relevant sectors (hospitals, academia, industry, patients) and integrating all disciplines: clinical and pre-clinical studies, physics, bioinformatics, computer science, statistics, mathematics, ICT infrastructure, ethical and societal aspects. HYPERBOOST will apply such a multi-sectoral and multidisciplinary approach to train the next generation of Early Stage Researchers (ESRs) who will be well equipped to tackle all urgent and complex clinical questions posed by modern multi-modality oncological treatments.
Objective
The objective of HYPERBOOST is therefore to train a new generation of creative and entrepreneurial professionals with the skills and expertise to coordinate, develop, apply and optimise advanced multi-modality cancer treatments. HYPERBOOST will also develop an advanced personalised treatment planning platform for hyperthermia based on extensive (pre)clinical data.
[1] Horsman MR et al. Clin Oncol (R Coll Radiol). 2007;19:418-26, and van den Tempel N et al. Int J Hyperthermia. 2016;32:446-54
[2] Krawczyk PM, et al. Proc Natl Acad Sci USA. 2011;108:9851-6, and Oei et al Radiat Oncol. 2015;10:165.
[3] Frey B, et al. Immunol Rev. 2017;280:231-248, Oei et al. Cancer Res 2015;75:5120-9, and Repasky EA, et al. Cancer Immunol Res. 2013;1:210-6
[4] Jones EL et al, Clin Cancer Res. 2004;10:4287-93, and Dewhirst MW et al. Int J Hyperthermia. 2005;21:779-9
[5] Horsman MR et al. Nat Rev Clin Oncol. 2012;9:674-87, and Overgaard et al. Radiother Oncol. 2011;100:22-32
[6] van Leeuwen CM, et al. Int J Hyperthermia. 2018;34:30-38
[7] van der Zee J et al. Lancet 2000;355:1119-25
